Describe How Crude Oil Is Separated Using Fractional Distillation
Crude oil vapour is put into a fractionating column at the bottom and rises upwards. Crude oil can be separated into different fractions using fractional distillation.
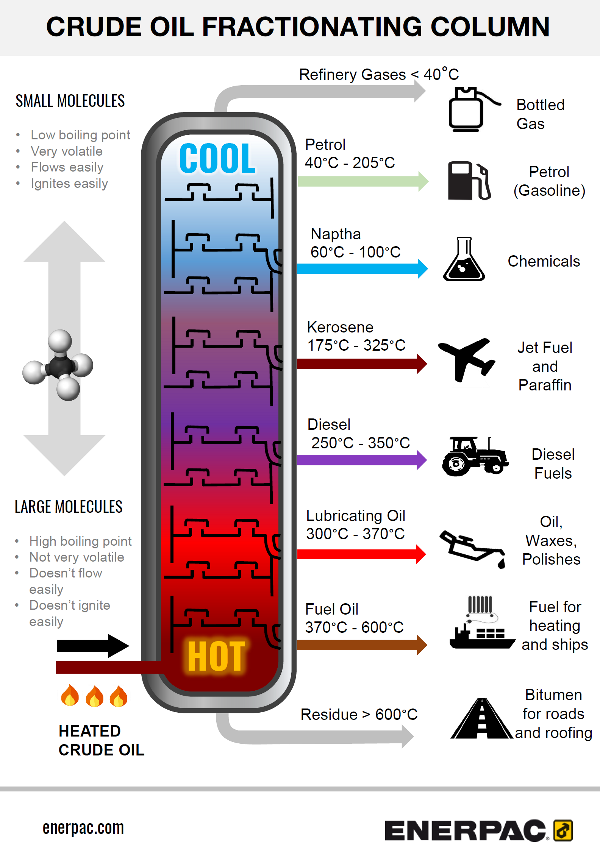
How Crude Oil Is Separated Into Fractions Enerpac Blog
Fracture is used to transform lengthy alkanes into small more functional hydrocarbons.

. Fractional distillation works because the different compounds within the crude oil mixture have different boiling points. But first the useful ones must be extracted from the crude oil and. The column is hot at the bottom and cool at the top.
The temperature is highest at the bottom of the column. Crude oil is heated until it evaporates. Gasoline and many other chemicals are produced from crude oil using fractional distillation.
Crude oil is separated into useful fractions by fractional distillation. The crude oil is evaporated and its vapours allowed to condense at different temperatures in the fractionating column. Describe how a fractional distillation column separates crude oil fractions.
It then goes into the tower. Crude oil is used to produce many useful materials. - cool condense hydrocarbons - at different temperatures.
Fractional distillation separates hydrocarbons using their different boiling points. Many useful products can be made from these hydrocarbons. Long-chain hydrocarbons condense at the bottom and.
The process of separating the various components of petroleum from one another is known as the refining of petroleum. The oil refining process. Crude oil is separated into fractions in a process called fraction distillation.
Decompose Page 16 of 32. Crude oil is heated and the oil evaporates. Crude oil is separated by fractional distillation.
The column is hot at the bottom and cool at the top. Fractions with different boiling points condense at different levels. - crude oil is heated and it becomes a vapour.
A tall fractionating column is fitted above the mixture with several condensers coming off at different heights. Crude oil is vapourised and fed into the bottom of the fractionating column. Evaporating burn During fractional distillation the compounds condense at different temperatures.
Explain how it works. Up to 24 cash back b Crude oil is separated into useful fractions by fractional distillation. Crude oil is separated by fractional distillation.
Heated crude oil enters a tall fractionating column which is hot at the bottom and gets cooler towards the top. During the fractional distillation of crude oil. Describe the separation of crude oil in fractional distillation.
The vapours then rise and the different hydrocarbons condense at their. Crude oil is heated until it evaporates. Because they have different boiling points the substances in crude oil can be separated using fractional distillation.
The vapours then rise and the different hydrocarbons condense at their specific boiling points allowing them to. It can be separated out into fractions using a fractionating column. The crude oil is evaporated and its vapours condense at different temperatures in the fractionating column.
Use the diagram to help you to explain how crude oil is separated into fractions. The crude oil mixture is separated in a distillation column. The steps of the process are.
As the vapours rise up the tower the temperature falls. - crude oil vapour is placed into the bottom of the fractionating column where it rises. The crude oil mixture is fed into the bottom of the column as a gas.
A The diagram shows some of the fractions produced from crude oil by fractional distillation. Crude oil is heated to vaporize the different hydrocarbons in a tank which is cool at the top and hot at the bottom. Crude oil is separated by fractional distillation.
Describe and explain how crude oil is separated into fractions by fractional distillation. Crude oil is evaporated and its vapours travel up the column condensing at different temperatures. Describe the process of separating crude oil using fractional distillation.
3 marks - heat evaporate the crude oil change to gas or vapour. Fractional distillation of crude oil. Describe and explain how the mixture of alkanes is separated by fractional distillation.
Crude oil is a mixture of many different hydrocarbons. Substances with high boiling points condense at the bottom and substances with lower boiling points condense on the way. As the vapour rises up the column the temperature falls.
Use the diagram to help you answer the question. By using the concept of boiling point during the fractional distillation process oil refinery workers can ensure the compounds of interest are correctly separated from a. This is done by a process called fractional distillation which is based on the fact that the different components of.
Different sized fractions condense at different heights because they have different boiling points. Up to 24 cash back Fractional distillation is used to separate the compounds in crude oil. Smaller molecules condense high up the tower.
Crude oil is separated into numerous chemicals such as gasoline diesel fuel and acetone are examples of fractional distillation. Fractional distillation procedure is the detachment of a mixture into its component parts. 2 cracking The first step in fractional distillation is displacing the crude oil.
- hydrocarbons with high boiling points will condense at the bottom long chain where it is collected as fractions. Crude oil is heated to vaporize the different hydrocarbons in a tank which is cool at the top and hot at the bottom. Fractional distillation Crude oil also called petroleum is a mixture of different hydrocarbons.
Fractional distillation separates a mixture into a number of different parts called fractions. Jun 7 2021 - Crude oil is a restricted asset. Fractional distillation The majority of our fuels and plastics are derived from oil.
The first thing that happens to crude oil is that it is fractionally distilled though trendy technology has precipitated a shift to chemical distillation by using excessive-tech chemical processes and reactions to separate the completely different grades of hydrocarbons though in some cases they need to combine hydrocarbons a process often called unification.
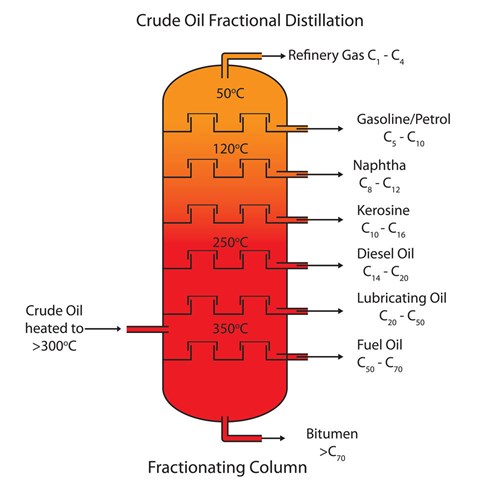
How To Demonstrate Fractional Distillation In A Science Class Philip Harris
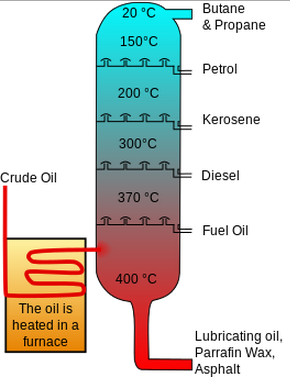
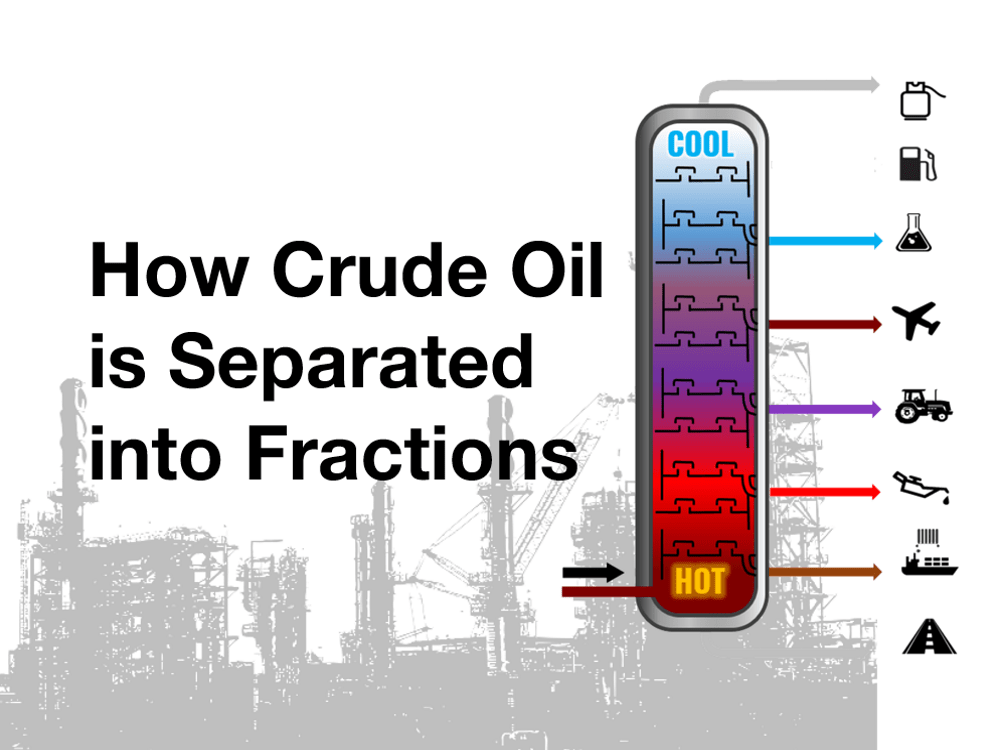
No comments for "Describe How Crude Oil Is Separated Using Fractional Distillation"
Post a Comment